RISKS MITIGATED
The WAK has a clear path to market
Three successful clinical trials and fast-tracked by the FDA.
Dialysis reimbursement from CMS is guaranteed by law. The
escalatingcosts have driven CMS to look for innovation ASAP!
Better performance data and R&D is further along than any competing technologies.
Protected by families of patents for the WAK device
THE CLINICAL OPPORTUNITY
The Wearable Artificial Kidney is a RARE opportunity to invest in a pre-market technology that has limited barrier to approval

Regulatory Risks Mitigated: FDA/CMS
Want It Approved

Unmet medical Need With
Pent Up Demand

Technology Risk Mitigated by
Previous Successes

World Renowned Medical Team
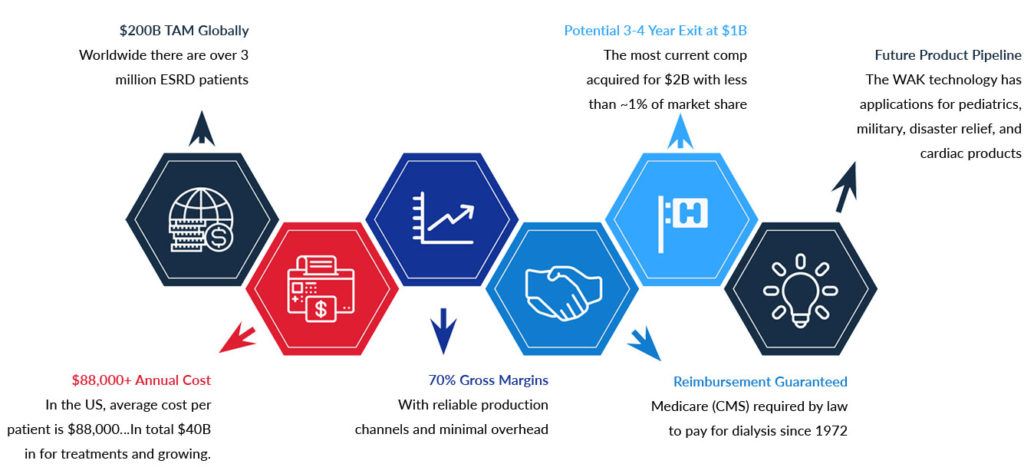
THE FINANCIAL OPPORTUNITY
The Wearable Artificial Kidney is a RARE opportunity to invest in a pre-market technology that will transform an entire market
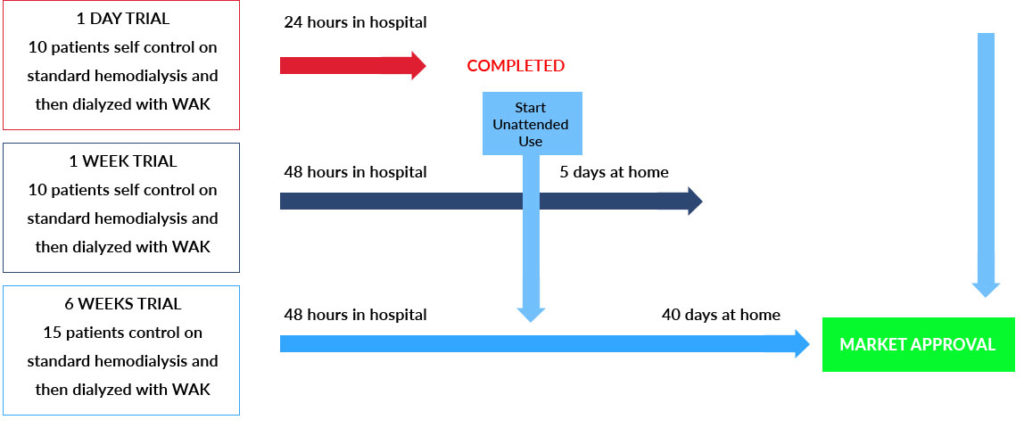
Only 2 remaining trials to FDA approval
INVESTOR CHECKLIST
Why you should have confidence investing in the WAK
